Fighting COVID has not only been a clinical challenge; equally, it has revealed many of the shortcomings of healthcare supply chain management. We have recently witnessed how the public health departments struggled to procure, distribute, and manage personal protective equipment (PPE) demand. And, for COVID vaccine logistics, this is going to be even bigger, complex, and mind-bogglingly challenging. Questions first arise with the vaccine’s initial supply shortage and the need for an equitable allocation and distribution mechanism to meet the priority access population demand. Finally, there is complying with all of the stringent ultra-cold environment requirements involved with shipping, storing, handling, and administering the shots.
The front running drugs being developed to combat the novel coronavirus through the immunization program are unique in nature (nucleic-acid vaccines in the form of DNA or RNA). They will likely require even more careful management to keep them efficacious and safe for administration. According to a recent Bloomberg article, members of supply chain industries know full well that they are not prepared to handle the vaccine’s unique transit and distribution needs. What will it take to get them ready? What tools do we need to arm our distribution and logistics industries with to manage the COVID vaccine supply chain efficiently?
Safe administration and monitoring of the COVID vaccine supply chain will require exceptional IT ability to track and monitor distribution and administration through the entire process—end-to-end. This is a first of its kind process, something that has never been required before in any vaccine program. This goes beyond mere shipping updates and delivery status reports. To maintain the drug’s efficacy and a constant stream of related vaccination supplies, supply chain managers and clinic personnel will have to consider the rigorous temperature control in the ultracold chain (all through their distribution process), high-demand inventory management, batch monitoring, and much more. Additionally, they must keep notifying the jurisdiction’s public health authorities and CDC at timely intervals.
So far in this series, we’ve gone over the overarching challenges, solution areas, and functional needs of a vaccination management system. Now, it’s time to look at the specific features included in the Infosys Vaccination Management (IVM) offering that bring visibility and real-time insights to the complete vaccine supply chain to ensure the highest possible integrity and avoidance of any discrepancies and intended compromise.
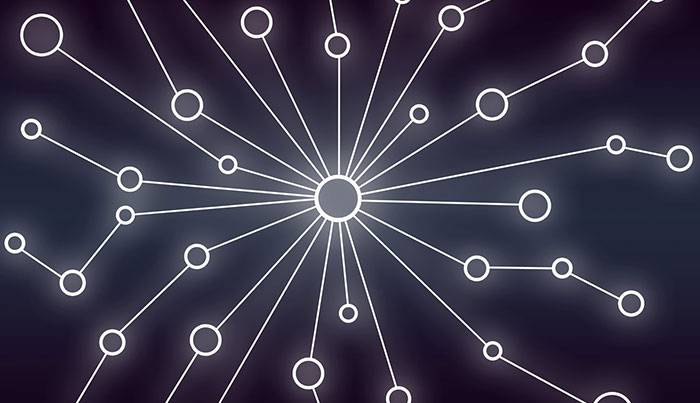
Blockchain
One of the foundational factors to creating a 360-degree view of vaccine inventory is blockchain technology. Blockchain will allow every stakeholder—vaccine administrators, pharmaceuticals, state regulatory organizations, and distributor—to follow the data of a single dose from source to target and for every stage of the supply chain. Understanding everything that’s happening and keeping track of it for every vaccination supply will be mission-critical for public health. As a real-time digital ledger, Blockchain makes that kind of secure, reliable, immutable, authentic data flow possible. This ensures the availability of quality vaccines from manufacturers to service-delivery levels in an urgent timeframe.
“The interest level for the pharmaceutical supply chain in blockchain would encompass tracking whichever components they need to be included in their vaccines, along with their own portion of the vaccine that they develop,” says Gina Parry, Distribution and Pharmaceutical Sales Manager at VAI. “The blockchain itself would enable confidence for everyone from the pharmaceutical supply chain, down to doctors and recipients of vaccines that it has been verified along every step, and safe for distribution.”
The Infosys Vaccine Management solution is designed with the infrastructure to work with blockchain data and provide…
Want to keep reading? Check out the full ebook here: The Complete Guide to COVID-19 Vaccine Management.



















































0 Comments